Fluorescent Primer Probe based chemistries are the core of real time pcr (qPCR). The most common fluorescent based chemistries are Taqman Probes and Molecular Beacons. Other fluorescent primer probe based chemistries includes Eclipse probes and Amplifluor, Scorpions, LUX, and BD QZyme primers.
All the above mentioned Primer - Probe based detection chemistries take advantage of Fluorescence Resonance Energy Transfer (FRET), or some other form of fluorescence quenching,which will ensure that the specific fluorescence is detected only in the presence of amplified product.
TaqMan Probe Chemistry
TaqMan = Taq Polymerase + PacMan
TaqMan assay includes an oligonucleotide probe called the TaqMan Probe, containing a fluorescent reporter dye at the 5' end and quencher at the 3' end. This oligonucleotide is developed in such a way that it will bind to downstream of the primer binding site in the target DNA molecule.
Molecular Beacons
In molecular beacon assay other than the two specific primers, a flourescently labelled oligonucleotide probe called the "molecular beacon", a dye-labelled oligonucleotide which forms a hairpin structure with stem and a loop.
Structure of Molecular Beacon
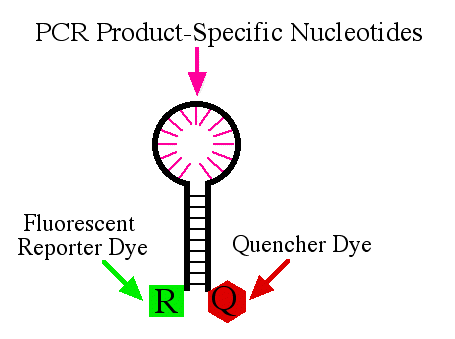
Molecular Beacon is usually 25-40 nucleotides long. molecular beacon has a flourescently labelled reporter at the 5' end and quencher at the 3' end. Typically a Molecular Beacon has:
1. Loop
Comparison of nine different real-time PCR chemistries for qualitative and quantitative applications in GMO detection.- Buh Gasparic M, Tengs T, La Paz JL, Holst-Jensen A, Pla M, Esteve T, Zel J, Gruden K.
 |
| A Typical Real Time PCR Amplification Plot (Ct Vs Fluorescence) |
All the above mentioned Primer - Probe based detection chemistries take advantage of Fluorescence Resonance Energy Transfer (FRET), or some other form of fluorescence quenching,which will ensure that the specific fluorescence is detected only in the presence of amplified product.
TaqMan Probe Chemistry
TaqMan = Taq Polymerase + PacMan
TaqMan assay includes an oligonucleotide probe called the TaqMan Probe, containing a fluorescent reporter dye at the 5' end and quencher at the 3' end. This oligonucleotide is developed in such a way that it will bind to downstream of the primer binding site in the target DNA molecule.
TaqMan assay utilizes the exonuclease activity of taq DNA polymerase, during the elongation step in the PCR, taq DNA polymerase displaces the bound reporter dye.
Due to the 5'-3' exonuclease activity of Taq DNA Polymerase, TaqMan probe will be removed / cleaved from the target strand, allowing primer extension to continue to the end of the template strand. Thus, inclusion of the probe does not inhibit the overall PCR process.
when the reporter and quencher are inn close proximity, the fluorescence emitted by the reporter dye will be readily quenched by the quencher, when the reporter is cleaved by the taq DNA polymerase, reporter moves away from the quencher thereby no quenching occurs, resulting in the emission of fluorescent signal.
TaqMan Assay Vs SYBR Green Assay
TaqMan Assay Vs SYBR Green Assay
Molecular Beacons
In molecular beacon assay other than the two specific primers, a flourescently labelled oligonucleotide probe called the "molecular beacon", a dye-labelled oligonucleotide which forms a hairpin structure with stem and a loop.
Structure of Molecular Beacon
1. Loop
2. Stem
3. 5' Flourophore
4. 3' Quencher
The Loop of molecular beacon is designed in such a way that it will hybridize to 15-30 nucleotide section of the target nucleic acid. As from the image above we can make out that the stem region is a set of complementary sequence which forms a stem like structure which keep the reporter and quencher close by. As a result of this hair pair pin form no fluorescence is detected from the reported because of the presence of quencher near to it. During the annealing step of the pcr reaction, molecular beacon binds to the target nucleic acid opening up the hairpin structure and as result the reporter and quencher moves father apart, so fluorescence is detected from the reporter. The amount of fluorescence emitted by the reporteron the molecular beacon is directly proportional to the amount of target in the reaction.
Difference between Molecular Beacon Assay and Taqman Assay
The main difference is that in molecular beacon assay Taq Polymerase used will lack 5'-3' exonuclease activity so here in molecular beacon assay molecular beacons are not destroyed. But in Taqman assay taq DNA polymerase with 5'-3' exonuclease activity is used which will cleave the probe.
Advantages of Molecular Beacons
Difference between Molecular Beacon Assay and Taqman Assay
The main difference is that in molecular beacon assay Taq Polymerase used will lack 5'-3' exonuclease activity so here in molecular beacon assay molecular beacons are not destroyed. But in Taqman assay taq DNA polymerase with 5'-3' exonuclease activity is used which will cleave the probe.
Advantages of Molecular Beacons
- Highly Specific compared to other Assays.
- Multiplexing Possible
- Allelic discrimination and identification
- Used for Single Nucleotide Polymorphisms.
Disadvantages of Molecular Beacons
- Difficult to design
- Unintended fluorescence can be produced if the hairpin opens into non-hairpin confirmation.
Hybridization Probe Assay
In Hybridization probe assay,In addition to two sequence specific primers, two sequence specific oligonucleotide probes are used.
There are two oligonucleotide probes, Probe 1 having donor dye at 3' end and the Probe 2 carries a acceptor dye at the 5' end. The dyes of donor and acceptor probes are selected in such a way that emission spectrum of the donor dye overlaps the excitation spectrum of the acceptor dye, where as the emission spectrum of the donor dye is separated from the emission spectrum of the acceptor dye. Excitation is performed at a wavelength specific to the donor dye, and the reaction is monitored at the emission wavelength of the acceptor dye. During the annealing step of PCR, the probes hybridize to their target sequences in a head-to-tail arrangement. This brings the fluorescent molecules into proximity, allowing fluorescence resonance energy transfer from donor to acceptor. The increasing amount of acceptor fluorescence is proportional to the amount of amplicon present.
Minor Groove Binder (MGB) Eclipse Probe
Eclipse Probe Assay will have two primers and a sequence specific oligonucleotide probe. The probe will bind to the specific target sequence, the specialty of MGB Eclipse Probe is that it has a 5' Quencher and a 3' Reporter. on the 5' end there is a minor groove binder.
In the Unhybridized state, reporter and quencher will be in close proximity resulting in the quenching of reporter. During the annealing step of pcr probe binds to the target sequence with the help of minor groove binder, once it binds reporter and quencher becomes far apart and fluorescence can be detected. The fluorescent signal is proportional to the amount of amplified product in the sample.
Amplifluor Chemistry
Amplifluor chemistry employ two target-specific primers and one universal primer called the UniPrimer. The first target-specific primer contains a 5' extension sequence called the Z-sequence that is also found at the 3' end of the UniPrimer. The UniPrimer forms a hairpin structure. A fluorescent reporter and a quencher are attached at the 5' and the 3' ends of the stem structure, respectively. In the hairpin conformation, the reporter fluorescence is quenched due to its proximity to the quencher. During the first amplification cycle, the first target-specific primer (with the Z-sequence) hybridizes to the template and is extended. During the second amplification cycle, the second target-specific primer is used to synthesize a new target template that contains a sequence complementary to the Z-sequence. The product from the second amplification cycle can then serve as the template for the UniPrimer. In the third amplification cycle, the extended UniPrimer serves as a template for the next amplification cycle. In the fourth cycle, extension of the template through the hairpin region of the UniPrimer causes the UniPrimer to open up and adopt a linear configuration, which allows the reporter to fluoresce. Exponential amplification using the second target-specific primer and the UniPrimer occurs in subsequent amplification cycles. The resulting fluorescent signal is proportional to the amount of amplified product in the sample.
Scorpion Primer Assay
Scorpions primer assays employ two primers, one of which serves as a probe and contains a stem-loop structure with a 5' fluorescent reporter and 3' quencher. The loop sequence of the Scorpions probe is complementary to an internal portion of the target sequence on the same strand. During the first amplification cycle, the Scorpions primer is extended and the sequence complementary to the loop sequence is generated on the same strand. The Scorpions probe contains a PCR blocker just 3' of the quencher to prevent read-through during the extension of the opposite strand. After subsequent denaturation and annealing, the loop of the Scorpions probe hybridizes to the target sequence by an intramolecular interaction, and the reporter is separated from the quencher. The resulting fluorescent signal is proportional to the amount of amplified product in the sample.
LUX (Light Upon Xtenstion) Primer Assay
LUX primer assays employ two primers, one of which is a hairpin-shaped primer with a fluorescent reporter attached near the 3' end. In the intact primer, the reporter is quenched by the secondary structure of the hairpin. During amplification, the LUX primer is incorporated into the product and the reporter fluoresces.
LUX Probe Invitrogen Manual
BD QZyme Primer Assay
BD QZyme primers employ a target-specific zymogene primer, a target-specific reverse primer, and a universal oligonucleotide substrate. The oligonucleotide contains a fluorescent reporter on the 5' end and a quencher on the 3' end. When oligonucleotide substrate is intact, the fluorescence of the reporter is quenched by the quencher due to their proximity. The zymogene primer contains a sequence that encodes a catalytic DNA. During the first amplification cycle, the zymogene primer is extended. In the second cycle, the product of the first cycle is used as the template by the target-specific reverse primer, which is extended to create a new target sequence containing a catalytic DNA region. In the subsequent annealing step, the fluorescently labeled oligonucleotide substrate hybridizes to the catalytic DNA sequence and is cleaved. This cleavage separates the reporter from the quencher, resulting in a fluorescent signal that is proportional to the amount of amplified product in the sample.
Scorpions primer assays employ two primers, one of which serves as a probe and contains a stem-loop structure with a 5' fluorescent reporter and 3' quencher. The loop sequence of the Scorpions probe is complementary to an internal portion of the target sequence on the same strand. During the first amplification cycle, the Scorpions primer is extended and the sequence complementary to the loop sequence is generated on the same strand. The Scorpions probe contains a PCR blocker just 3' of the quencher to prevent read-through during the extension of the opposite strand. After subsequent denaturation and annealing, the loop of the Scorpions probe hybridizes to the target sequence by an intramolecular interaction, and the reporter is separated from the quencher. The resulting fluorescent signal is proportional to the amount of amplified product in the sample.
LUX (Light Upon Xtenstion) Primer Assay
LUX primer assays employ two primers, one of which is a hairpin-shaped primer with a fluorescent reporter attached near the 3' end. In the intact primer, the reporter is quenched by the secondary structure of the hairpin. During amplification, the LUX primer is incorporated into the product and the reporter fluoresces.
LUX Probe Invitrogen Manual
BD QZyme Primer Assay
BD QZyme primers employ a target-specific zymogene primer, a target-specific reverse primer, and a universal oligonucleotide substrate. The oligonucleotide contains a fluorescent reporter on the 5' end and a quencher on the 3' end. When oligonucleotide substrate is intact, the fluorescence of the reporter is quenched by the quencher due to their proximity. The zymogene primer contains a sequence that encodes a catalytic DNA. During the first amplification cycle, the zymogene primer is extended. In the second cycle, the product of the first cycle is used as the template by the target-specific reverse primer, which is extended to create a new target sequence containing a catalytic DNA region. In the subsequent annealing step, the fluorescently labeled oligonucleotide substrate hybridizes to the catalytic DNA sequence and is cleaved. This cleavage separates the reporter from the quencher, resulting in a fluorescent signal that is proportional to the amount of amplified product in the sample.
References
Minor Groove Binder-Conjugated DNA Probes for Quantitative DNA Detection by Hybridization-Triggered Fluorescence - I.A. Afonina, M.W. Reed, E. Lusby, I.G. Shishkina, and Y.S. Belousov
Epoch Biosciences, Bothell, WA, and Synthetic Genetics, San Diego, CA, USA
Use of self-quenched, fluorogenic LUX primers for gene expression profiling. - Kusser W.
Use of self-quenched, fluorogenic LUX primers for gene expression profiling. - Kusser W.
Multiplex real-time PCR assay using Scorpion probes and DNA capture for genotype-specific detection of Giardia lamblia on fecal samples. - Ng CT, Gilchrist CA, Lane A, Roy S, Haque R, Houpt ER.
Technical Resources:
Sigma Aldrich
Life Technologies
Life Technologies
Biorad Laboratories
Gene Quantification




No comments:
Post a Comment