Transfection is the process of introduction of foreign DNA into the nucleus of eukaryotic cell. The cells which has incorporated exogenous DNA are called transfectants.
There are two types of Transfection possible,
- Transient and
- Stable Transfection.
Stable transfectants will have the foreign DNA incorporated into the genome.
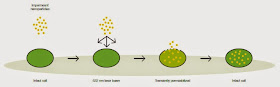
Here is the overview of transient Transfection protocol.
Transfection Methodologies:
Following are the methodologies that are used for transfection, each of the below strategies has its own advantages and disadvantages, so one need to choose the suitable method based on the application.
- Reagent Based
- Instrument Based
- Viral Mediated
1. Transfection - Reagent Based
Reagent based methods are simple, The reagent used, neutralizes the negative charge and condenses the DNA for effective uptake.
Reagent based methods are simple, The reagent used, neutralizes the negative charge and condenses the DNA for effective uptake.
As the cell membrane is negatively charged, -vely charged DNA molecules will be repelled from the cells, for the cells to take up DNA the net charge need to be positive, that is the reason why reagents are used. The main principle of using reagents is to give the DNA a net positive charge either by reagents binding to the DNA or by forming complexes with the DNA.
- Calcium Phosphate
- Lipids
- Cationic Polymers
 |
| Overview of Reagent Based Transfection |
Calcium Phosphate mediated Transfection
 The mechanism of DNA uptake by calcium phosphate mediated method is not well understood. In this method, the DNA to transfected is mixed with calcium chloride, then this is added slowly to a buffered saline or phosphate solution. Incubating this mixture with the cells to be transfected. The cells will take up the DNA by endocytic pathways,
The mechanism of DNA uptake by calcium phosphate mediated method is not well understood. In this method, the DNA to transfected is mixed with calcium chloride, then this is added slowly to a buffered saline or phosphate solution. Incubating this mixture with the cells to be transfected. The cells will take up the DNA by endocytic pathways,
Lipid Mediated Transfection - Lipofection
Polar lipids formed of highly positive charge head groups attached to hydrophobic tails.
Electrostatic Interaction between positive charges of the head groups of cationic lipids and negatively charged phosphate groups of the DNA are the main forces that allow DNA to be spontaneously associated with the cationic lipids.
Cationic Lipids used in Transfection
Examples of Lipids used in Transfection are:
- DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine)
- DOTMA (1,2-di-O-octadecenyl-3-trimethylammonium propane)
- DOTAP (1,2-dioleoyl-3-trimethylammonium-propane)
Lipoplexes
Lipoplexes are organized lipid molecules in the form of micelle or liposomes, which when complexed with DNA can be used effectively for transfection.
The net positive charge of the lipoplexes are effective for transfection.
Reagents for Transfection are also made of helper lipids.

Helper lipids in conjunction with cationic lipids forms structures called liposomes, they can effectively encapsulate DNA.
Cationic polymers differ from cation lipids as they do not have hydrophobic moeity cationic polymers can more efficiently condense DNA.
There are 3 different types of cationic polymers used for transfection
- Linear
- Branched
- Spherical
Cationic Polymers includes PEI and dendrimers.
This complex has net positive charge, are very effective in transfection.
DEAE - Dextran
DEAE dextran is a cationic polymer that tightly associated with negatively charged nucleic acid.
The Positively charge polymer:DNA complex comes into close association with negatively charged membrane.
Charge – Transfection complex should have a net cationic (+) charge.
Positive surface charge density – Zeta Potential (+mV).
Charge ratio:
Reagent Concentration in Nitrogen residues (mM) / DNA Concentration in phosphate moeities (mM)
*N/P ratio is very important for optimization.
Transfection - Instrument Based
Nucleic acids are transported mechanically into the cells, following are the commonly used methods for instrument based transfection.
- Electroporation
- Biolistic Technology
- Microinjection
- Laserfection / Optoinjection
Electroporation
The procedure involves placing the cell suspension and the foreign material (eg: Plamsid) to be
transferred into plastic or glass cuvette.The cuvettes used here are specially meant for electroporation, they have aluminium electrodes at the sides. The voltage and capacitance is set and the cuvette inserted into the electroporator.
Once after electroporation, few ml medium is added , and is incubated at the the culture's optimal temperature for an hour or more to allow recovery of the cells.The transformed cells can be screened out using selectable markers in the plasmid (usaually antibiotic resisitance genes are used).
Advantages and Disadvantages of Electroporation
Electroporation has the following advantages and disadvantages
Advantages:
Versatility: Electroporation is effective with nearly all cell and species types.
Efficiency: A large majority of cells take in the target DNA or molecule.
Advantages and Disadvantages of Electroporation
Electroporation has the following advantages and disadvantages
Advantages:
Versatility: Electroporation is effective with nearly all cell and species types.
Efficiency: A large majority of cells take in the target DNA or molecule.
Small Scale: The amount of DNA required is smaller as compared with other methods.
Disadvantages:
Cell Damage: If the electric pulses are given for long time or with more intensity, some pores may become too large or fail to close after membrane discharge causing cell damage or rupture
Nonspecific Transport: The transport of material into and out of the cell during the time of electropermeability is relatively nonspecific. This may result in an ion imbalance that could later lead to improper cell function and cell death.
Biolistic Transformation / Gene Gun
Biolistic Transformation is the transfer of nucleic acid into cells via high velocity nucleic acid coated microparticles
Laserfection/Optoinjection
This procedure uses laser light to transiently permeabilize cells in very short time. Various substances can be efficiently opoinjected including ions, small molecules, dextrans,plasmids, proteins, etc
Viral Mediated Transfection
Viral Vectors
Advantages and Disadvantages of different Transfection Methods
Evaluating Transfection Performance: Reporter systems
- Green Fluorescent Protein
- Luciferase Reporter
- Beta – Galactosidase
- Secreted Alkaline Phosphatase
Direct Visualization of Nucleic acid delivery
Fluorescent labels – Cyanine Dyes, Fluorescein
Epitope Tags
Covalent linkage of dye to the DNA.
- Media
- Nucleic Acid
- Complexing Time
- Cell Culture condition / Confluency
- Harvesting Time
Media
Culture media plays a major role in transfection efficiency. Cell culture media mostly supplemented with serum. For efficient transfection transfection complex should be made in serum free media. The reason for serum free media usage for transfection complex formation is that, the serum has nucleases in it which can chew up the nucleic acids,
Antibiotics also should be avoided in the transfection complex formation media, as the antibiotics are charged, they can interfere in the transfection complex formation and there by reducing the transfection efficiency.
Other charged compounds like polyanions (eg: heparin sulfate) should be avoided and also surfactants like pluronic F68, mostly used as antifoaming agent, should be avoided.
The quality of nucleic acid used for transfection is also important. The plasmid purity or the 260/280 ratio of the plasmid used for transfection should be greater than 1.8.
Most of the times plasmid preps gets contaminated with Endotoxins (endotoxins are toxic to cells),The endotoxin contaminated DNA inhibits the transfection or will yield very low transfection efficiency.
Complexing Time
Complexing time is yet another important factor which need to be optimized for better transfection efficiency. If the the complexing time is too then the complex formed will be big, which will be difficult for the cells to take up, there by reducing the transfection efficiency.
Ideal complex formation time is 15 - 30 mins, but this need to be experimented as the different cell lines will behave differently, complex formation time above 30 minutes will have low transfection efficiency.
For mRNA transfection ideal complex formation time will be 5 - 10 mins.
Cell Culture Conditions
- Adherent cell confluency affects transfection.
- Low confluency – 25% - Not good.
- 50 – 70% confluency is good for transfection.
- At greater than 90% confluency, cells will not take up DNA to nuclei because of the dissociation of nuclear membrane.
References
- Life Technologies , Technical Resources
- Biorad, Technical Resources
- Qiagen, Technical Resources
- Mirus Bio, Technical Resoources














No comments:
Post a Comment